Dual enzyme-responsive “turn-on” fluorescence sensing systems based on in situ formation of 7-hydroxy-2-iminocoumarin scaffolds†
Abstract
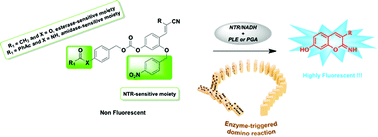
A new strategy for the simultaneous fluorogenic detection of two distinct enzyme activities namely hydrolase (amidase or esterase) and reductase is described. This innovative biosensing method is based on the powerful “covalent-assembly” principle that involves in situ synthesis of a fluorophore from a non-fluorescent caged precursor and through domino reactions triggered by the two analytes of interest. To establish this approach, penicillin G acylase (PGA) (or pig liver esterase (PLE)) and nitroreductase (NTR) were chosen as model enzymes, and original bis-O-protected 2,4-dihydroxycinnamonitrile derivatives acting as dual-reactive probes readily convertible to highly fluorescent 7-hydroxy-2-iminocoumarin scaffolds upon reacting with the two selected enzymes were synthesised. The two phenolic groups available within the core structure of these probes play a pivotal role in generating iminocoumarin scaffold through an intramolecular cyclisation reaction (hydroxyl group in C-2 position) and in enhancing its push–pull character (hydroxyl group in C-4 position). Their orthogonal and temporary protection with two different enzyme-labile masking groups is the cornerstone in the design of this novel class of fluorogenic “turn-on” probes. Their evaluation using fluorescence-based in vitro assays and HPLC-fluorescence/-MS analyses have enabled us both to demonstrate the claimed activation mechanism (in particular the specific order in which the two enzymes react with the probe) and to highlight the potential utility of these advanced chemical tools in multi-analyte sensing applications.

 Please wait while we load your content...
Please wait while we load your content...