Versatile synthesis of oxime-containing acyclic nucleoside phosphonates – synthetic solutions and antiviral activity†
Abstract
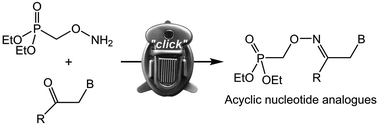
New oxime-containing acyclic nucleoside phosphonates 9-{2-[(phosphonomethyl)oximino]ethyl}adenine (1), -guanine (2) and 9-{2-[(phosphonomethyl)oximino]propyl}adenine (3) with wide spectrum activity against different types of viruses were synthesized. The key intermediate, diethyl aminooxymethylphosphonate, was obtained by the Mitsunobu reaction. Modified conditions for the by-product separation (without chromatography and distillation) allowed us to obtain 85% yield of the aminooxy intermediate. The impact of DBU and Cs2CO3 on the N9/N7 product ratio for adenine and guanine alkylation was studied. A convenient procedure for aminooxy group detection was found. The synthesized phosphonates were tested and they appeared to display moderate activity against different types of viruses (HIV, herpes viruses in cell cultures, and hepatitis C virus in the replicon system) without toxicity up to 1000 μM.

 Please wait while we load your content...
Please wait while we load your content...