Degradation of HaloTag-fused nuclear proteins using bestatin-HaloTag ligand hybrid molecules†
Abstract
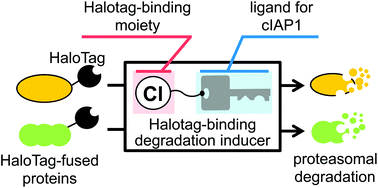
We have developed a protein knockdown technology using hybrid small molecules designed as conjugates of a ligand for the target protein and a ligand for ubiquitin ligase cellular inhibitor of apoptosis protein 1 (cIAP1). However, this technology has several limitations. Here, we report the development of a novel protein knockdown system to address these limitations. In this system, target proteins are fused with HaloTag to provide a common binding site for a degradation inducer. We designed and synthesized small molecules consisting of alkyl chloride as the HaloTag-binding degradation inducer, which binds to HaloTag, linked to BE04 (2), which binds to cIAP1. Using this system, we successfully knocked down HaloTag-fused cAMP responsive element binding protein 1 (HaloTag-CREB1) and HaloTag-fused c-jun (HaloTag-c-jun), which are ligand-unknown nuclear proteins, in living cells. HaloTag-binding degradation inducers can be synthesized easily, and are expected to be useful as biological tools for pan-degradation of HaloTag-fused proteins.

 Please wait while we load your content...
Please wait while we load your content...