Organic base-promoted enantioselective electrophilic cyanation of β-keto esters by using chiral phase-transfer catalysts†
Abstract
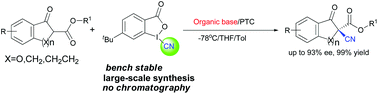
Highly enantioselective cyanation of β-keto esters using hypervalent iodine(III) as the electrophilic cyanating reagent induced by cinchona alkaloid-based chiral quaternary ammonium salt was demonstrated. Organic bases, especially DMAP, in the chiral phase-transfer catalysis were used to obtain high ees.

 Please wait while we load your content...
Please wait while we load your content...