Tryptophan prenyltransferases showing higher catalytic activities for Friedel–Crafts alkylation of o- and m-tyrosines than tyrosine prenyltransferases†
Abstract
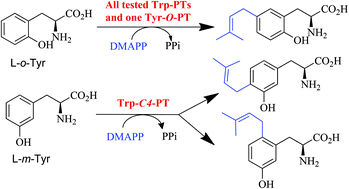
Tryptophan prenyltransferases FgaPT2, 5-DMATS, 6-DMATSSv and 7-DMATS catalyse regiospecific C-prenylations on the indole ring, while tyrosine prenyltransferases SirD and TyrPT catalyse the O-prenylation of the phenolic hydroxyl group. In this study, we report the Friedel–Crafts alkylation of L-o-tyrosine by these enzymes. Surprisingly, no conversion was detected with SirD and three tryptophan prenyltransferases showed significantly higher activity than another tyrosine prenyltransferase TyrPT. C5-prenylated L-o-tyrosine was identified as a unique product of these enzymes. Using L-m-tyrosine as the prenylation substrate, product formation was only observed with the tryptophan prenyltransferases FgaPT2 and 7-DMATS. C4- and C6-prenylated derivatives were identified in the reaction mixture of FgaPT2. These results provided additional evidence for the similarities and differences between these two subgroups within the DMATS superfamily in their catalytic behaviours.

 Please wait while we load your content...
Please wait while we load your content...