Ring closing metathesis reactions of α-methylene-β-lactams: application to the synthesis of a simplified phyllostictine analogue with herbicidal activity†
Abstract
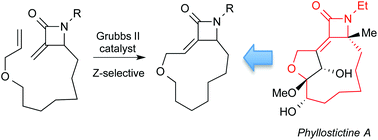
Ring closing metathesis (RCM) reactions of α-methylene-β-lactams are used to construct strained 11- and 12-membered macrocycles that mimic key structural elements of phyllostictine A. The highest yield and stereoselectivity was achieved making 12-membered macrocycle Z-19 with use of a p-methoxyphenyl group on the lactam nitrogen. Interestingly, substrate concentration had an important influence on the stereochemical course of the reaction. A simplified analogue produced using this approach displays phytotoxic activity against Chlamydomonas reinhardtii suggesting that the α-methylene-β-lactam subunit is responsible, at least in part, for the herbicidal activity of phyllostictine A.

 Please wait while we load your content...
Please wait while we load your content...