Copper-free arylation of 3,3-disubstituted allylic halides with triazene-softened aryl Grignard reagents†
Abstract
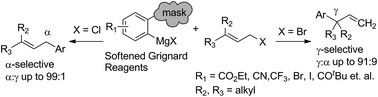
A copper-free allylic arylation reaction between 3,3-disubstituted allylic halides and triazene-softened aryl Grignard reagents has been developed. This protocol presents a direct and efficient way to construct both α- or γ-isomers with high regioselectivity under environmentally benign conditions. Various functional groups can be tolerated in the reaction and the products are of high value for multiple synthetic applications. The α- and γ-isomers can be converted to the corresponding 3H-indole and indole derivatives in multigram scale respectively.

 Please wait while we load your content...
Please wait while we load your content...