Copper-catalyzed asymmetric allylation of chiral N-tert-butanesulfinyl imines: dual stereocontrol with nearly perfect diastereoselectivity†
Abstract
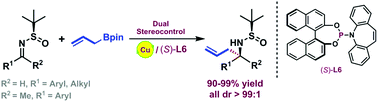
Copper-catalyzed asymmetric allylation of chiral N-tert-butanesulfinyl imines has been described. Dual stereocontrol, through the combination of a chiral auxiliary and a chiral copper complex, has played an important role in achieving the nearly perfect diastereoselectivities (all dr > 99 : 1), especially for ketimine substrates.

 Please wait while we load your content...
Please wait while we load your content...