Applying the prodrug strategy to α-phosphonocarboxylate inhibitors of Rab GGTase – synthesis and stability studies†‡
Abstract
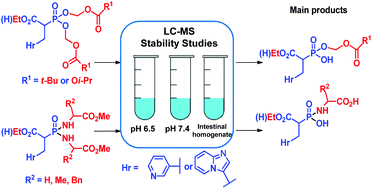
Fourteen novel prodrug-like analogs of two highly ionic phosphonocarboxylate inhibitors of Rab geranylgeranyl transferase were synthesized and preliminary assessment of their chemical and enzymatic stability was evaluated in buffers (pH 6.5 and 7.4) and rat intestinal homogenate (pH 6.5). Both acidic groups in phosphonocarboxylates were subject to modification. Phosphonic acid was protected either as bis(acyloxyalkyl) ester or phosphonodiamidate derived from amino acids. The carboxylic acid group was either left unchanged or was studied as ethyl ester. The compounds exhibited favorable stability in physiologically relevant pH (t1/2 above 18 h), while in intestinal homogenate they showed a large variety of half-lives (from 5 minutes to over 150 hours). LC MS studies have shown that the main product of decomposition under studied conditions resulted from cleavage of one of the ester (for acyloxyalkyl analogs) or amide (for phosphonodiamidate) bonds with phosphorus.

 Please wait while we load your content...
Please wait while we load your content...