Frontier orbitals and transition states in the oxidation and degradation of l-ascorbic acid: a DFT study†
Abstract
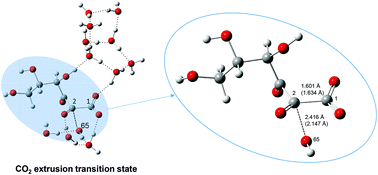
DFT calculations were carried out to investigate reaction paths of L-ascorbic acid (AAH2), hydroxyl radicals and water clusters. Frontier-orbital analyses were also performed to examine the regioselectivity of the OH˙ addition. Transition states of the electrolytic dissociation of AAH2 and intermediate carboxylic acids were found to have very small activation energies through proton transfers along hydrogen bonds. The ionized species (anions) are subject to the electrophilic attack of OH˙. The elementary processes of AAH2 → A˙− → dehydroascorbic acid → diketogulonic acid → threonic, oxalic, xylonic and lyxonic acids were investigated and discussed. The processes involved in the conversion of dehydroascorbic acid into a bicyclic hemiketal were also examined as a side-chain participating reaction. The oxidation and degradation of vitamin C up to threonic acid were described mainly as a donor (AAH2)–acceptor (OH˙) reaction.

 Please wait while we load your content...
Please wait while we load your content...