Tuning of the colour and chemical stability of model boranils: a strong effect of structural modifications†
Abstract
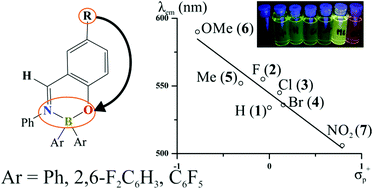
A series of diarylborinic complexes with salicydeneaniline ligands bearing various functional groups at the 6-position have been synthesized in high yields by applying a straightforward one-pot multicomponent protocol. UV-Vis measurements revealed the influence of electronic character of substituents on the observed maximum of emission (λem). This has been confirmed by a relatively strong linear correlation (R2 = 0.92) of λem with Hammett σp+ constants. Such a correlation was investigated using a QTAIM analysis of the charge density distribution. Absorption and emission bands for the obtained systems span between 390–437 nm and between 506–590 nm, respectively, with quantum yields reaching 17%. Time-dependent UV-Vis absorption measurements revealed that diphenylborinic salicydeneaniline complexes undergo slow degradation in solution under ambient conditions. In contrast, the use of a naphthalene-based chromophore or the introduction of fluorinated phenyl groups at the boron atom resulted in stable systems.

 Please wait while we load your content...
Please wait while we load your content...