Enantioselective carbolithiation of S-alkenyl-N-aryl thiocarbamates: kinetic and thermodynamic control†
Abstract
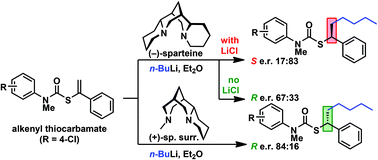
The addition of n-butyllithium to alkenylthiocarbamates in the presence of (−)-sparteine or the (+)-sparteine surrogate leads to asymmetric carbolithiation, and returns enantiomerically enriched thiocarbamate derivatives of secondary thiols. In THF, with the (+)-sparteine surrogate, in situ aryl migration leads to an enantiomerically enriched tertiary thiol derivative. Remarkably, the two pseudoenantiomeric chiral ligands do not always give enantiomeric products, probably as a result of a complex interplay of kinetic and thermodynamic control. In situ IR and NMR studies of a stable, hindered lithiated thiocarbamate demonstrated its chemical and configurational stability over a period of hours at 0 °C.

 Please wait while we load your content...
Please wait while we load your content...