Synthesis and evaluation of a boronate-tagged 1,8-naphthalimide probe for fluoride recognition†
Abstract
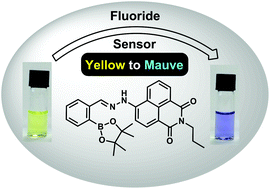
A biocompatible fluoride receptor has been developed where the interaction between the boronic acid ester and amine (NH) results in fluoride ion selectivity and enhanced fluorescence quenching.
- This article is part of the themed collection: Supramolecular Chemistry in Water

 Please wait while we load your content...
Please wait while we load your content...