The direct electrophilic cyanation of β-keto esters and amides with cyano benziodoxole†
Abstract
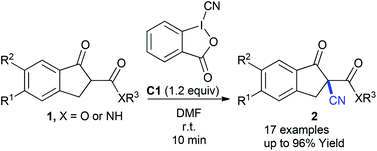
The direct electrophilic α-cyanation of β-keto esters and amides has been developed using a hypervalent iodine benziodoxole-derived cyano reagent. The procedure is accomplished within 10 min and without the use of any catalyst in DMF, at room temperature. Thus, the highly functionalized quaternary carbon-centered nitriles were produced in high to excellent yields.

 Please wait while we load your content...
Please wait while we load your content...