Synthesis of magnetic cobalt ferrite nanoparticles with controlled morphology, monodispersity and composition: the influence of solvent, surfactant, reductant and synthetic conditions†
Abstract
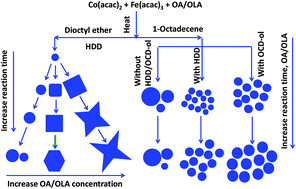
In our present work, magnetic cobalt ferrite (CoFe2O4) nanoparticles have been successfully synthesised by thermal decomposition of Fe(III) and Co(II) acetylacetonate compounds in organic solvents in the presence of oleic acid (OA)/ oleylamine (OLA) as surfactants and 1,2-hexadecanediol (HDD) or octadecanol (OCD-ol) as an accelerating agent. As a result, CoFe2O4 nanoparticles of different shapes were tightly controlled in size (range of 4–30 nm) and monodispersity (standard deviation only at ca. 5%). Experimental parameters, such as reaction time, temperature, surfactant concentration, solvent, precursor ratio, and accelerating agent, in particular, the role of HDD, OCD-ol, and OA/OLA have been intensively investigated in detail to discover the best conditions for the synthesis of the above magnetic nanoparticles. The obtained nanoparticles have been successfully applied for producing oriented carbon nanotubes (CNTs), and they have potential to be used in biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...