Multifunctional gold nanorods for selective plasmonic photothermal therapy in pancreatic cancer cells using ultra-short pulse near-infrared laser irradiation†
Abstract
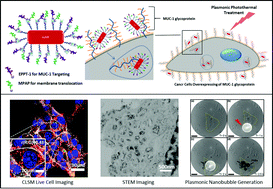
Gold nanorods (AuNRs) have attracted considerable attention in plasmonic photothermal therapy for cancer treatment by exploiting their selective and localized heating effect due to their unique photophysical properties. Here we describe a strategy to design a novel multifunctional platform based on AuNRs to: (i) specifically target the adenocarcinoma MUC-1 marker through the use of the EPPT-1 peptide, (ii) enhance cellular uptake through a myristoylated polyarginine peptide (MPAP) and (iii) selectively induce cell death by ultra-short near infrared laser pulses. We used a biotin–avidin based approach to conjugate EPPT-1 and MPAP to AuNRs. Dual-peptide (EPPT-1 + MPAP) labelled AuNRs showed a significantly higher uptake by pancreatic ductal adenocarcinoma cells when compared to their single peptide or avidin conjugated counterparts. In addition, we selectively induced cell death by ultra-short near infrared laser pulses in small target volumes (∼1 μm3), through the creation of plasmonic nanobubbles that lead to the destruction of a local cell environment. Our approach opens new avenues for conjugation of multiple ligands on AuNRs targeting cancer cells and tumors and it is relevant for plasmonic photothermal therapy.

 Please wait while we load your content...
Please wait while we load your content...