Tuning the reorganization energy of electron transfer in supramolecular ensembles – metalloporphyrin, oligophenylenevinylenes, and fullerene – and the impact on electron transfer kinetics†
Abstract
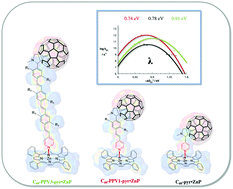
Oligo(p-phenylenevinylene) (oPPV) wires of various lengths featuring pyridyls at one terminal and C60 moieties at the other, have been used as molecular building blocks in combination with porphyrins to construct a novel class of electron donor–acceptor architectures. These architectures, which are based on non-covalent, directional interactions between the zinc centers of the porphyrins and the pyridyls, have been characterized by nuclear magnetic resonance spectroscopy and mass spectrometry. Complementary physico-chemical assays focused on the interactions between electron donors and acceptors in the ground and excited states. No appreciable electron interactions were noted in the ground state, which was being probed by electrochemistry, absorption spectroscopy, etc.; the electron acceptors are sufficiently decoupled from the electron donors. In the excited state, a different picture evolved. In particular, steady-state and time-resolved fluorescence and transient absorption measurements revealed substantial electron donor–acceptor interactions. These led, upon photoexcitation of the porphyrins, to tunable intramolecular electron-transfer processes, that is, the oxidation of porphyrin and the reduction of C60. In this regard, the largest impact stems from a rather strong distance dependence of the total reorganization energy in stark contrast to the distance independence seen for covalently linked conjugates.

 Please wait while we load your content...
Please wait while we load your content...