Mechanistic studies on the indole prenyltransferases
Abstract
Covering: up to 2014
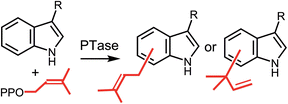
Prenylated indole alkaloids comprise a large and structurally diverse family of natural products that often display potent biological activities. In recent years a large family of prenyltransferases that install prenyl groups onto the indole core have been discovered. While the vast majority of these enzymes are evolutionarily related and share a common protein fold, they are remarkably versatile in their ability to catalyze reverse and normal prenylations at all positions on the indole ring. This highlight article will focus on recent studies of the mechanisms utilized by indole prenyltransferases. While all of the prenylation reactions may follow a direct electrophilic aromatic substitution mechanism, studies of structure and reactivity suggest that in some cases prenylation may first occur at the nucleophilic C-3 position, and subsequent rearrangements then generate the final product.

 Please wait while we load your content...
Please wait while we load your content...