Influence of the solvent on the complexation of Cm(iii) and Eu(iii) with nPr–BTP studied by time-resolved laser fluorescence spectroscopy
Abstract
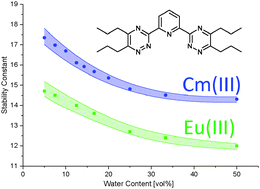
The complexation of Cm(III) and Eu(III) with 2,6-bis(5,6-dipropyl-1,2,4-triazin-3-yl)pyridine (nPr–BTP) in methanol–water mixtures with varying water content is investigated by TRLFS. For both metal ions, the exclusive formation of the 1 : 3 complex is observed in each solvent mixture. Stability constants are determined by analysis of the fluorescence spectra. As a result, an immense influence of the water content is found. The stability constant of the [Cm(nPr–BTP)3]3+ complex increases by three orders of magnitude when the water content in methanol–water mixtures is reduced (log β3 = 14.3 ± 0.1 at 50 vol% water content; log β3 = 17.4 ± 0.4 at 5 vol% water content). Due to the preferential solvation of Cm(III) by water in methanol–water mixtures, the increase is moderate at water contents between 50 vol% and 20 vol% and becomes steeper at lower water contents. For the [Eu(nPr–BTP)3]3+ complex, an analogous evolution of the stability constant is observed (log β3 = 12.0 ± 0.1 at 50 vol% water content; log β3 = 14.7 ± 0.4 at 5 vol% water content). Therefore, the difference between the stability constants of the Cm(III) and Eu(III) 1 : 3 complexes is constant at all solvent mixtures investigated (Δlog β3 = 2.3 ± 0.3) which means that the selectivity of the N-donor ligand is not influenced by the solvent.

 Please wait while we load your content...
Please wait while we load your content...