Interaction of Nd(iii) and Cm(iii) with borate in dilute to concentrated alkaline NaCl, MgCl2 and CaCl2 solutions: solubility and TRLFS studies
Abstract
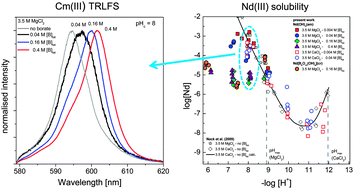
The interaction of lanthanides and trivalent actinides with borate in dilute to concentrated alkaline NaCl, MgCl2 and CaCl2 solutions was investigated at 22 ± 2 °C by a comprehensive series of solubility experiments with Nd(OH)3(am), and complemented with Cm(III)–TRLFS studies (TRLFS: time resolved laser fluorescence spectroscopy) under analogous pH and ionic strength conditions. Although there was clear evidence of borate complexation in the pH range of 8.5 to 10, overall no significant increase in Nd(III) solubility occurred in any of the investigated salt systems in the presence of [B]tot ≤ 0.4 M, compared with analogous borate-free solutions. On the contrary, a significant decrease in Nd(III) concentration was observed at pHc ≤ 9 in NaCl and MgCl2 systems with [B]tot ≥ 0.16 M (diluted salt systems) or [B]tot ≥ 0.04 M (concentrated salt systems). This observation, together with a clear change in the slope of the solubility curve and the further confirmation by XPS analyses, indicates the transformation of Nd(OH)3(am) into a so far unknown Nd(III)–borate solid phase with significantly lower solubility. Similar Nd(III) concentrations in the aqueous phase are obtained in undersaturation solubility experiments conducted with a synthesized crystalline phase Nd[B9O13(OH)4](cr). TRLFS confirmed the formation of aqueous Cm(III)–borate complexes in dilute to concentrated NaCl and MgCl2 systems at pHc = 8 and [B]tot ≥ 0.04 M. Two different Cm(III)–borate species are proposed based on the peak shift of the spectra, although the resulting fluorescence emission bands do not allow the definition of an unequivocal chemical model for this system. TRLFS also shows that no Cm(III)–borate complexes form under hyperalkaline conditions (pHc = 12), due to the stronger competition posed by hydrolysis and the predominance of weakly coordinating B(OH)4− in the aqueous phase. These results show the impact of An(III)–borate interactions on An(III) speciation and highlight the hitherto unknown role of borate in the immobilization of trivalent actinides under repository-relevant conditions due to the formation of borate-bearing solid phases with significantly lower solubility than the corresponding hydroxides.

 Please wait while we load your content...
Please wait while we load your content...