Investigating the generation of hydrogen sulfide from the phosphonamidodithioate slow-release donor GYY4137†
Abstract
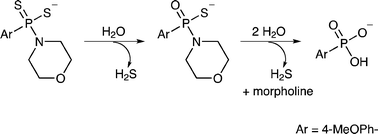
A combination of NMR spectroscopy, mass spectrometry and chemical synthesis was used to elucidate the two-step hydrolytic decomposition pathway of the slow-release hydrogen sulfide (H2S) donor GYY4137 and the key decomposition product was also prepared by an independent synthetic route. The (dichloromethane-free) sodium salt of the phosphonamidodithioate GYY4137 was also produced as a pharmaceutically more acceptable salt. In contrast with GYY4137 and its sodium salt, the decomposition product did not generate H2S or exert cytoprotective or anti-inflammatory effects in oxidatively stressed human Jurkat T-cells and LPS-treated murine RAW264.7 macrophages. The decomposition product represents a useful control compound for determining the biological and pharmacological effects of H2S generated from GYY4137.


 Please wait while we load your content...
Please wait while we load your content...