Synthesis and biological evaluation of 2,3-diarylthiophene analogues of combretastatin A-4†
Abstract
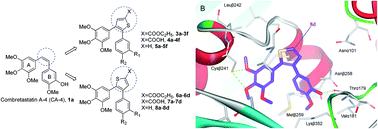
A series of novel 2,3-diarylthiophene analogues of combretastatin A-4 (CA-4) were designed with a rigid thiophene moiety to retain the cis-olefin configuration of CA-4 and were subsequently synthesised. All of the target compounds were evaluated for their in vitro anti-proliferative activities. Among these compounds, 5f and 8d exhibited superior potency against different tumour

 Please wait while we load your content...
Please wait while we load your content...