Analysis of fast protein phosphorylation kinetics in single cells on a microfluidic chip†
Abstract
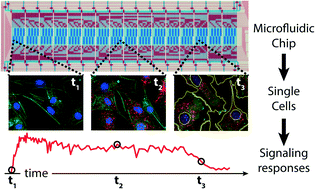
In the present study, we developed a microfluidic large-scale integration (mLSI) platform for the temporal and chemical control of cell cultures to study fast kinetics of protein phosphorylation. For in situ protein analysis the mLSI chip integrates the Proximity Ligation Assay (PLA). To investigate cell-signaling events with a time resolution of a few seconds we first engineered and optimized the fluidic layout of the chip with 128 individual addressable cell culture chambers. The functionality of the cell culture operations and PLA is demonstrated by the determination of the minimum cell sample size for obtaining robust quantitative PLA signals at the single-cell level. We show that at least 350 cells per assay condition are required to statistically evaluate single cell PLA data. In the following we used the PLA chip with over 500 hundred cells per condition to record sequential phosphorylation reactions of the canonical protein kinase within the Akt pathway, which is activated in various human cancer types. This was achieved by stimulating mouse fibroblast cell cultures with either the platelet-derived growth factor (PDGF) or insulin-like growth factor (IGF-1). Fluidic cell stimulation pulses of 5 seconds were followed by precisely time shifted cell fixation pulses to obtain a temporal resolution of 10 seconds. PLA was then performed on all fixed arrays of cell cultures to extract the characteristic phosphorylation times at the single cell level for either the PDGF, or IGF-1 receptor and the Akt and GSK3β kinases. Characteristic phosphorylation times for the receptors were between 13 and 35 seconds, whereas for downstream kinases between 25 and 200 seconds. Thus we could reveal a molecular order of the phosphorylation reactions during the signal transduction through the Akt pathway. In dependence of the stimulus we found a temporal difference for the characteristic phosphorylation time of 20 and 150 seconds for the Ser-473 and Thr-308 residues on the Akt kinase, respectively. Temporal alteration of sequential phosphorylation reactions on Akt has been proposed as molecular mechanism to differentiate between stimuli and biophysically determined in the present study.

 Please wait while we load your content...
Please wait while we load your content...