A multigram-scale lower E-factor procedure for MIBA-catalyzed direct amidation and its application to the coupling of alpha and beta aminoacids†
Abstract
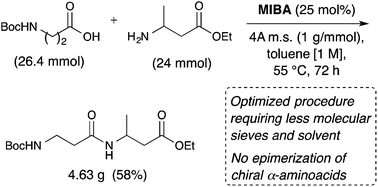
The development of direct and atom-economical amidation methods is of high priority because of the importance of amides and peptides as components of pharmaceuticals and commodity chemicals. This article describes the identification of more economical and more practical conditions for direct amidation of carboxylic acids and amines using the MIBA catalyst (5-methoxy-2-iodophenylboronic acid, 6) and its application to the coupling of α- and β-amino acid derivatives. It is now possible to use half of the quantity of molecular sieves prescribed in the original procedure, at a higher concentration leading to a reduction of waste and a substantially improved E-factor. This procedure was validated in the multigram scale preparation of prototypical amides, including aminoacids, using toluene as the solvent. Because of substrate inhibition of the catalyst with monoprotected α-aminoacids, the use of doubly-protected N-phthaloyl α-aminoacids or α-azidoacids is required in order to produce dipeptide products in moderate yields. β-Aminoacids do not suffer from this problem, and Boc-β-aminoacids can be coupled successfully. Unlike other boronic acid catalysts, 6 is active under ambient and low-heat conditions, which helps prevent any epimerization of chiral α-aminoacid derivatives.

 Please wait while we load your content...
Please wait while we load your content...