Synthesis of five- and six-membered heterocycles by dimethyl carbonate with catalytic amounts of nitrogen bicyclic bases†
Abstract
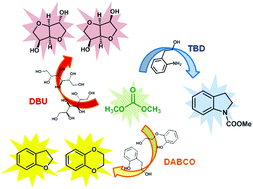
A catalytic amount of a nitrogen bicyclic base, i.e., DABCO, DBU or TBD, is effective for the one-pot synthesis of heterocycles from 1,4-, 1,5-diols and 1,4-bifunctional compounds via dimethyl carbonate chemistry under neat conditions. Nitrogen bicyclic bases that were previously shown to have enhanced the reactivity of DMC in methoxycarbonylation reaction by a BAc2 mechanism are herein used for the first time as efficient catalysts for cyclization reactions encompassing both BAc2 and BAl2 pathways. This synthesis procedure was also applied to a large scale synthesis of cyclic sugars, isosorbide and isomannide, starting from D-sorbitol and D-mannitol, respectively. The resulting anhydro sugar alcohols were obtained as pure crystalline compounds that did not require any further purification or crystallization.

 Please wait while we load your content...
Please wait while we load your content...