Iodine-catalyzed regioselective sulfenylation of imidazoheterocycles in PEG400†
Abstract
An iodine-catalyzed sulfenylation of imidazo[1,2-a]-pyridines, -pyrimidines, and [1,2-b]pyridazines is herein described with various thiophenols using hydrogen peroxide as an oxidizing agent in PEG400. The method enabled the formation of 3-arylthioimidazoheterocycles in moderate to excellent yields under metal-free conditions. Several functional groups were well tolerated under our optimized conditions.
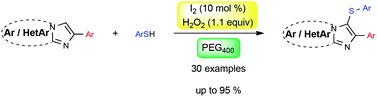

 Please wait while we load your content...
Please wait while we load your content...