Formation of disinfection by-products during ballast water treatment with ozone, chlorine, and peracetic acid: influence of water quality parameters†
Abstract
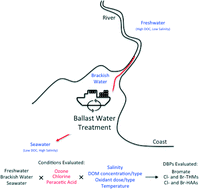
As ocean-going ships begin implementing chemical disinfection to treat ballast water, the potential formation of disinfection by-products (DBPs) is an important issue of concern. This is especially critical since ballast waters are often saline, and the information regarding DBP formation under these conditions is limited. This study exposed representative ballast waters (synthetically-made and natural freshwaters, brackish waters, and seawaters) to ozone, free chlorine, and peracetic acid (PAA) treatment where various water quality parameters and treatment conditions were varied to assess DBP formation. DBPs including bromate, trihalomethanes (THMs), and haloacetic acids (HAAs) were affected by changes in salinity (especially bromide concentration), dissolved organic matter (DOM) concentration/type, and oxidant type/dose. Temperature effects (22 ± 2 °C or 4 ± 2 °C) were limited for THMs and HAAs formation but were greater for bromate formation (only ozone) in waters containing high bromide levels. Interestingly, bromate formation during ozonation was rapid (complete formation in <15 min) but not linearly proportional to the bromide concentration when its molar concentration exceeded the molar ozone dose. Its formation was well predicted using a simplified kinetic model, previously applied to freshwaters, incorporating known reactions of ozone with bromide to form bromate. Kinetic studies indicated that increased bromide concentrations in brackish waters/seawaters led to higher Br-DBPs formation (brominated THMs and HAAs) for all oxidants tested. These included bromoform (CHBr3), dibromoacetic acid (DBAA), and tribromoacetic acid (TBAA), which formed for all three oxidants but also dibromochloromethane (CHBr2Cl) and bromoacetic acid (MBAA) which formed during chlorination and PAA treatment or for only PAA treatment, respectively. Approximately 50% formation of the DBPs occurred within 24 h of the typical 5 day ballast water treatment holding time. The oxidant dosage experiments, when normalized to the DOM concentration, indicated that for the brackish waters/seawaters, formation typically overlapped each other and that outliers were driven by the specific UV absorbance (SUVA) values. In addition, formation of CHBr3 and TBAA was found to correlate well with each other for all three oxidants, indicating that they are derived from similar DOM precursors while CHBr3 and TBAA correlated to DBAA formation to a lesser degree, similar to observations found in chlorinated freshwaters.

 Please wait while we load your content...
Please wait while we load your content...