Impact of Pahokee Peat humic acid and buffer identity on goethite aggregation and reactivity†
Abstract
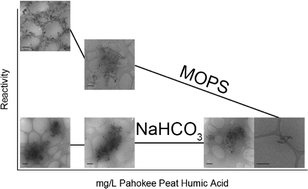
Natural organic matter (NOM) has been shown to strongly influence both reactions occurring at the solid–liquid interface and particle aggregation. Ferrous iron bound to iron oxides, such as goethite, can serve as a reductant towards environmental contaminants. Little is known, however, about how NOM affects reactivity and aggregation of iron oxide nanoparticles in aqueous conditions. Here, the rate of 4-chloronitrobenzene (4-ClNB) reduction is used to assess the reactivity of Fe(II) on goethite, and this reactivity is tracked as a function of increasing NOM concentration, using either 3-(N-morpholino)propanesulfonic acid (MOPS) or bicarbonate buffer at pH 7. Pahokee Peat humic acid (PPHA), extracted from an agricultural peat soil, was selected to simulate the NOM present in groundwater. Generally speaking, the 4-ClNB degradation rate decreases with increasing PPHA concentration. Characterization of the goethite nanoparticles after repeated cycles of 4-ClNB degradation demonstrated that oxidative crystal growth occurred mainly along the goethite c-axis, reaction rates progressively slowed with each cycle, and no new phases precipitated. Finally, bicarbonate buffer, a model for groundwater, dramatically affects both aggregation and reaction rates, with reaction rates an order of magnitude slower and aggregates substantially larger than observed in suspensions prepared using MOPS.

 Please wait while we load your content...
Please wait while we load your content...