Extraordinarily efficient photocatalytic hydrogen evolution in water using semiconductor nanorods integrated with crystalline Ni2P cocatalysts†
Abstract
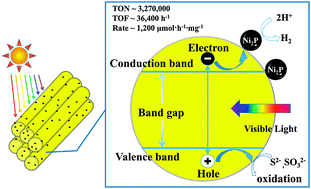
Photocatalytic hydrogen evolution via water splitting is an attractive scientific and technological goal to address the increasing global demand for clean energy and to reduce the climate change impact of CO2 emission. Although tremendous efforts have been made, hydrogen production by a robust and highly efficient system driven by visible light still remains a significant challenge. Herein we report that nickel phosphide, as a cocatalyst to form a well-designed integrated photocatalyst with one-dimensional semiconductor nanorods, highly improves the efficiency and durability for photogeneration of hydrogen in water. The highest rate for hydrogen production reached ∼1200 μmol h−1 mg−1 based on the photocatalyst. The turnover number (TON) reached ∼3 270 000 in 90 hours with a turnover frequency (TOF) of 36 400 for Ni2P, and the apparent quantum yield was ∼41% at 450 nm. The photoinduced charge transfer process was further confirmed by steady-state photoluminescence spectra and time-resolved photoluminescence spectra. Such extraordinary performance of a noble-metal-free artificial photosynthetic hydrogen production system has, to our knowledge, not been reported to date.
- This article is part of the themed collection: 2015 most accessed Energy & Environmental Science articles

 Please wait while we load your content...
Please wait while we load your content...