A highly active nanostructured metallic oxide cathode for aprotic Li–O2 batteries†
Abstract
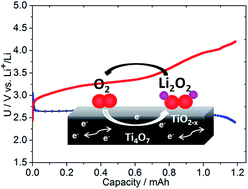
The aprotic lithium–oxygen cell is based on the reversible reduction of oxygen on a cathode host to form lithium peroxide, and has received much attention in the last few years owing to its promise to offer increased electrochemical energy density beyond that provided by traditional Li-ion batteries. Carbon has been extensively utilized as a host, but it reacts with Li2O2 to form an insulating layer of lithium carbonate resulting in high overpotentials on charge. Establishing a stable, and conductive interface at the porous cathode is a major challenge that has motivated a search for non-carbonaceous cathode materials. Very few suitable materials have been discovered so far. Here we report on the synthesis of the metallic Magnéli phase Ti4O7 with a crystallite size between 10–20 nm, and show that a cathode fabricated from this material greatly reduces the overpotential compared to carbon. Oxidation of lithium peroxide on charge starts just above 3 V, comparable to gold and TiC, and the majority (∼65%) of oxygen release occurs in the 3–3.5 V window vs. Li+/Li as determined by on-line electrochemical mass spectrometry. Ti4O7 is much lighter and lower cost than gold, easy to prepare, and provides a controlled interface. X-ray photoelectron spectroscopy measurements show that a conductive, self-passivating substoichiometric metal oxide layer is formed at the surface which is important for stability.

 Please wait while we load your content...
Please wait while we load your content...