Lipophilic M(α,α′-OC5H11)8phthalocyanines (M = H2 and Ni(ii)): synthesis, electronic structure, and their utility for highly efficient carbonyl reductions†
Abstract
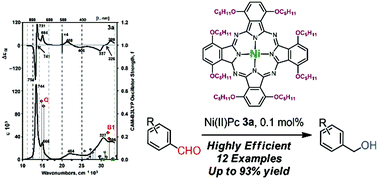
A lipophilic and electron-rich phthalocyanine (α,α′-n-OC5H11)8-H2Pc and its nickel(II) complex (α,α′-n-OC5H11)8-Ni(II)Pc have been synthesized and characterized. Detailed analyses of the electronic structure were carried out by spectroscopy, electrochemistry, spectroelectrochemistry, and TD-DFT calculations. A series of experiments demonstrate that the (α,α′-n-OC5H11)8-Ni(II)Pc complex can be used as a catalyst for highly efficient carbonyl reductions.

 Please wait while we load your content...
Please wait while we load your content...