An explicit account of solvation is essential for modeling Suzuki–Miyaura coupling in protic solvents†
Abstract
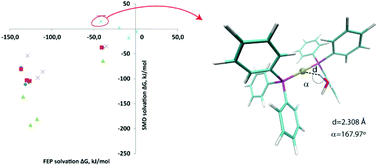
We compared explicit and implicit solvation approaches in modeling the free energy profile of the final step of Suzuki–Miyaura coupling. Both approaches produced similar ΔG≠ in all the studied solvents (benzene, toluene, DMF, ethanol, and water). Solvation free energies of individual reaction components reasonably correlated for explicit and implicit models in aprotic solvents (RMSE = 30–50 kJ mol−1, R2 > 0.71). However for ethanol and water the correlation was poor. We attributed this difference to the formation of the Pd⋯H–O hydrogen bond with Pd(PPh3)2 which was surprisingly observed in explicit modeling. Further QM calculations of the Pd(PPh3)2–H2O system confirmed the direction (Pd⋯H) and stability of this bonding. Therefore we stress the need for considering explicit solvation for modeling Pd-catalyzed reactions in protic solvents.

 Please wait while we load your content...
Please wait while we load your content...