Synthesis, DNA binding, cellular DNA lesion and cytotoxicity of a series of new benzimidazole-based Schiff base copper(ii) complexes†
Abstract
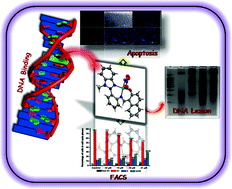
A series of new benzimidazole containing compounds 2-((1-R-1-H-benzimidazol-2-yl)phenyl-imino)naphthol HL1–3 (R = methyl, ethyl or propyl, respectively) have been synthesized by Schiff base condensation of 2-(1-R-1-H-benzo[d]imidazol-2-yl)aniline and 2-hydroxy-1-naphthaldehyde. The reactions of HL1–3 with Cu(NO3)2·2.5H2O led to the corresponding copper(II) complexes [Cu(L)(NO3)] 1–3. All the compounds were characterized by conventional analytical techniques and, for 1 and 3, also by single-crystal X-ray analysis. The interactions of complexes 1–3 with calf thymus DNA were studied by absorption and fluorescence spectroscopic techniques and the calculated binding constants (Kb) are in the range of 3.5 × 105 M−1–3.2 × 105 M−1. Complexes 1–3 effectively bind DNA through an intercalative mode, as proved by molecular docking studies. The binding affinity of the complexes decreases with the size increase of the N-alkyl substituent, in the order of 1 > 2 > 3, which is also in accord with the calculated LUMOcomplex energies. They show substantial in vitro cytotoxic effect against human lung (A-549), breast (MDA-MB-231) and cervical (HeLa) cancer cell lines. Complex 1 exhibits a significant inhibitory effect on the proliferation of the A-549 cancer cells. The antiproliferative efficacy of 1 has also been analysed by a DNA fragmentation assay, fluorescence activated cell sorting (FACS) and nuclear morphology using a fluorescence microscope. The possible mode for the apoptosis pathway of 1 has also been evaluated by a reactive oxygen species (ROS) generation study.

 Please wait while we load your content...
Please wait while we load your content...