Abstract
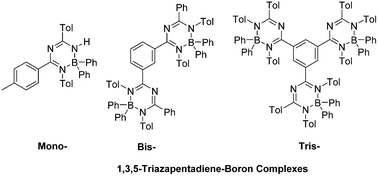
A series of novel benzene centered mono-, bis- and tris-1,3,5-triazapentadiene ligands 6a–e was synthesized and investigated with respect to their reactivity towards triphenylborane. The resulting blue-fluorescent boron complexes 14a–e with a six-membered ring chelate structure show excellent thermal and chemical stability. All title compounds were completely characterized including X-ray diffraction studies for 14a–c and 14e. Whereas the absorption spectra of all three classes of compounds are similar, the fluorescence spectra show distinct differences. Thus, the emission spectra of 14a,b show Stokes shifts of 4100–6700 cm−1 with low quantum yields both in solution and in the solid state. However, the more bulky compounds 14c–e show markedly larger molar extinction coefficients and smaller bathochromic shifts compared to 14a,b. For all compounds, we observe significantly more intense red-shifted fluorescence in the solid state compared to that in dichloromethane solutions. For the interpretation of the absorption properties TD-DFT studies were performed based on DFT geometry optimizations.

 Please wait while we load your content...
Please wait while we load your content...