1,1-Carboboration to tellurium–boron intramolecular frustrated Lewis pairs†
Abstract
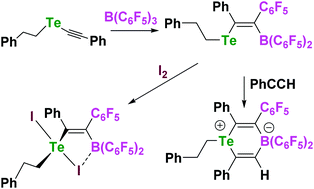
The tellurium acetylide Ph(CH2)2TeCCPh reacts with boranes via 1,1-carboboration to give a series of borylated vinyl telluroethers. The product Ph(CH2)2TeC(Ph)C(C6F5) B(C6F5)2 reacts with phenylacetylene and iodine to give novel tellurium–boron heterocyclic compounds.

 Please wait while we load your content...
Please wait while we load your content...