Coordination geometry of lead carboxylates – spectroscopic and crystallographic evidence†
Abstract
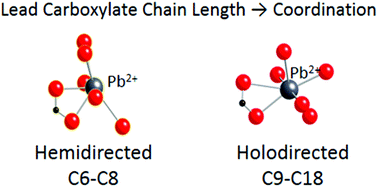
Despite their versatility, only a few single-crystal X-ray structures of lead carboxylates exist, due to difficulties with solubility. In particular, the structures of long-chain metal carboxylates have not been reported. The lone electron pair in Pb(II) can be stereochemically active or inactive, leading to two types of coordination geometries commonly referred to as hemidirected and holodirected structures, respectively. We report 13C and 207Pb solid-state NMR and infrared spectra for a series of lead carboxylates, ranging from lead hexanoate (C6) to lead hexadecanoate (C18). The lead carboxylates based on consistent NMR parameters can be divided in two groups, shorter-chain (C6, C7, and C8) and longer-chain (C9, C10, C11, C12, C14, C16, and C18) carboxylates. This dichotomy suggests two modes of packing in these solids, one for the short-chain lead carboxylates and one for long-chain lead carboxylates. The consistency of the 13C and 207Pb NMR parameters, as well as the IR data, in each group suggests that each motif represents a structure characteristic of each subgroup. We also report the single-crystal X-ray diffraction structure of lead nonanoate (C9), the first single-crystal structure to have been reported for the longer-chain subgroup. Taken together the evidence suggests that the coordination geometry of C6–C8 lead carboxylates is hemidirected, and that of C9–C14, C16 and C18 lead carboxylates is holodirected.

 Please wait while we load your content...
Please wait while we load your content...