The catalytic mechanism of A-type dye-decolourising peroxidase BsDyP: neither aspartate nor arginine is individually essential for peroxidase activity†
Abstract
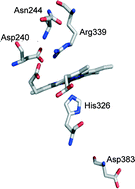
BsDyP from Bacillus subtilis belongs to the new dye-decolourising peroxidase (DyP) family. Here, we use transient kinetics to provide details on the catalytic cycle of BsDyP. The reaction of BsDyP with H2O2 exhibits saturation behaviour consistent with a two-step mechanism involving the formation of an E–H2O2 intermediate (K1 = (12 ± 1) × 10−6 M) followed by the formation of Compound I (k1 = 22 ± 1 s−1). We demonstrate that k1obs is pH-dependent and controlled by an ionisable group with a pKa of 4.3, suggesting the involvement of distal Asp. The reaction of Compound I with guaiacol obeys second-order kinetics (k3′ = (0.21 ± 0.01) × 106 M−1 s−1) while the reaction of Compound II with guaiacol shows saturation kinetics (K4 = (22 ± 5) × 10−6 M and k4 = 0.13 ± 0.01 s−1) and is the rate-limiting step in the BsDyP catalytic cycle. Furthermore, we use transient and steady-state kinetics as well as spectroscopic and electrochemical approaches to investigate the role of distal Asp240, Arg339 and Asn244 and proximal Asp383 residues in BsDyP. All mutations of distal residues affect particularly the K1 (and Km) for H2O2 leading to catalytic efficiencies (kcat/Km) of only one to two orders of magnitude lower than those in the wild-type. Notably, a significant improvement in the catalytic efficiency for reducing substrates is observed in the variants. We conclude that the Asp and Arg residues are important for the proper binding of H2O2 to the haem but none is individually indispensable for promoting H2O2 (de)protonation and O–O bond cleavage. The obtained kinetic data suggest an important role of distal Asn in modulating the acid–base catalysis of BsDyP. Our findings contribute to the establishment of structural determinants of DyPs that underlie their mechanistic properties; this has implications for their potential in biotechnological applications and sheds more light on the subfamily-dependent features of these enzymes.

 Please wait while we load your content...
Please wait while we load your content...