Non-alternant non-benzenoid kekulenes: the birth of a new kekulene family
Abstract
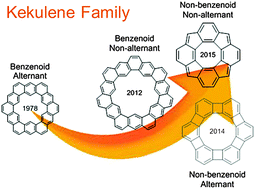
“Kekulene” is a doughnut-like shaped polycyclic aromatic hydrocarbon consisting of cyclically arrayed benzene rings. It has attracted a great deal of theoretical interest because it is regarded as an ideal model to study conjugation circuits of π electrons, i.e. whether they delocalize locally in benzene rings or globally throughout the molecule. Though kekulene was synthesized in 1978, it was the only known compound of this class of compounds for a long time. Recently, new kekulene-related molecules, septulene, which is a non-alternant benzenoid hydrocarbon, and a tetracyclopentatetraphenylene (TCPTP) derivative belonging to non-alternant non-benzenoid hydrocarbons, were synthesized. This article presents theoretical and experimental aspects of kekulene-related molecules focusing on the viewpoint of conjugation circuits by classifying them into three types: benzenoid kekulenes including kekulene itself and septulene, yet unknown anti-kekulene and non-alternant non-benzenoid kekulenes represented by TCPTP.
- This article is part of the themed collection: Challenges in Aromaticity: 150 Years after Kekulé’s Benzene

 Please wait while we load your content...
Please wait while we load your content...