Multi-platinum anti-cancer agents. Substitution-inert compounds for tumor selectivity and new targets
Abstract
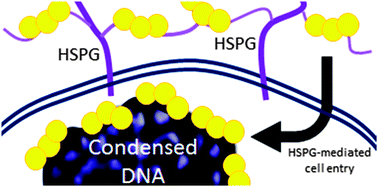
This tutorial review summarizes chemical, biophysical and cellular biological properties of formally substitution-inert “non-covalent” polynuclear platinum complexes (PPCs). We demonstrate how modulation of the pharmacological factors affecting platinum compound cytotoxicity such as cellular accumulation, reactivity toward extracellular and intracellular sulfur–ligand nucleophiles and consequences of DNA binding is achieved to afford a profile of biological activity distinct from that of covalently-binding agents. The DNA binding of substitution-inert complexes is achieved by molecular recognition through minor groove spanning and backbone tracking of the phosphate clamp. In this situation, the square-planar tetra-am(m)ine Pt(II) coordination units hydrogen bond to phosphate oxygen OP atoms to form bidentate N–O–N motifs. The modular nature of the polynuclear compounds results in high-affinity binding to DNA and very efficient nuclear condensation. These combined effects distinguish the phosphate clamp as a third mode of ligand–DNA binding, discrete from intercalation and minor-groove binding. The cellular consequences mirror those of the biophysical studies and a significant portion of nuclear DNA is compacted, a unique effect different from mitosis, senescence or apoptosis. Substitution-inert PPCs display cytotoxicity similar to cisplatin in a wide range of cell lines, and sensitivity is indifferent to p53 status. Cellular accumulation is mediated through binding to heparan sulfate proteoglycans (HSPG) allowing for possibilities of tumor selectivity as well as disruption of HSPG function, opening new targets for platinum antitumor agents. The combined properties show that covalently-binding chemotypes are not the unique arbiters of cytotoxicity and antitumor activity and meaningful antitumor profiles can be achieved even in the absence of Pt–DNA bond formation. These dual properties make the substitution-inert compounds a unique class of inherently dual-action anti-cancer agents.
- This article is part of the themed collection: Molecular Medicine and Cancer

 Please wait while we load your content...
Please wait while we load your content...