Recent advances in the synthesis of nitrogen heterocycles via radical cascade reactions using isonitriles as radical acceptors
Abstract
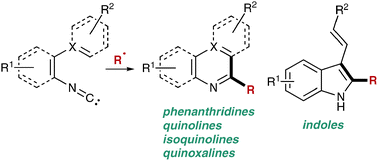
Nitrogen heterocycles belong to a highly important class of compounds which are found in various natural products, biologically active structures, and medicinally relevant compounds. Therefore, there is continuing interest in the development of novel synthetic methods for the construction of nitrogen containing heterocycles. Recently, radical insertion reactions into isonitriles have emerged as an efficient and powerful strategy for the construction of nitrogen heterocycles, such as phenanthridines, indoles, quinolines, quinoxalines, and isoquinolines. This review highlights recent advances in this fast growing research area and also includes important pioneering studies in this area.

 Please wait while we load your content...
Please wait while we load your content...