Defect chemistry and lithium transport in Li3OCl anti-perovskite superionic conductors†
Abstract
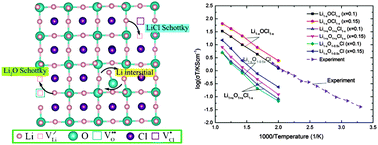
Lithium-rich anti-perovskites (LiRAPs) are a promising family of solid electrolytes, which exhibit ionic conductivities above 10−3 S cm−1 at room temperature, among the highest reported values to date. In this work, we investigate the defect chemistry and the associated lithium transport in Li3OCl, a prototypical LiRAP, using ab initio density functional theory (DFT) calculations and classical molecular dynamics (MD) simulations. We studied three types of charge neutral defect pairs, namely the LiCl Schottky pair, the Li2O Schottky pair, and the Li interstitial with a substitutional defect of O on the Cl site. Among them the LiCl Schottky pair has the lowest binding energy and is the most energetically favorable for diffusion as computed by DFT. This is confirmed by classical MD simulations, where the computed Li ion diffusion coefficients for LiCl Schottky systems are significantly higher than those for the other two defects considered and the activation energy in LiCl deficient Li3OCl is comparable to experimental values. The high conductivities and low activation energies of LiCl Schottky systems are explained by the low energy pathways of Li between the Cl vacancies. We propose that Li vacancy hopping is the main diffusion mechanism in highly conductive Li3OCl.

 Please wait while we load your content...
Please wait while we load your content...