Novel pnicogen bonding interactions with silylene as an electron donor: covalency, unusual substituent effects and new mechanisms
Abstract
An analogue of carbene, singlet silylene (H3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)2Si, was paired with the mono-substituted phosphines XH2Y (X = P, As, and Sb; Y = F, Cl, Br, and I) to form unconventional pnicogen-bonded complexes. All structures have Cs symmetry except the Sb complex, showing a deviation from this symmetry due to the coexistence of H⋯H interactions. The P and As complexes have different geometries from conventional pnicogen-bonded ones because the Y–X⋯Si line shows a large deviation from the molecular plane composed of two N atoms and one Si atom of (H3P
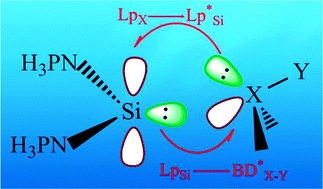
N)2Si, was paired with the mono-substituted phosphines XH2Y (X = P, As, and Sb; Y = F, Cl, Br, and I) to form unconventional pnicogen-bonded complexes. All structures have Cs symmetry except the Sb complex, showing a deviation from this symmetry due to the coexistence of H⋯H interactions. The P and As complexes have different geometries from conventional pnicogen-bonded ones because the Y–X⋯Si line shows a large deviation from the molecular plane composed of two N atoms and one Si atom of (H3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)2Si. This deviation can be attributed to a new formation mechanism of the pnicogen bond due to the combined result of the LPSi → BD*X–Y and LPX → LP*Si orbital interactions. Generally, the pnicogen bond becomes stronger in the order of F < Cl < Br < I and weaker in the order of P > As > Sb, exhibiting an unexpected substitution effect and dependence on the nature of the pnicogen atom. These orders are inconsistent with the MEP on the X atom but can be better explained by the above orbital interactions. The Si⋯X interaction displays a character of covalent or partially covalent interaction, evidenced by the high interaction energy of −59.9 to −105.4 kJ mol−1 as well as the negative energy density and the great charge transfer.
N)2Si. This deviation can be attributed to a new formation mechanism of the pnicogen bond due to the combined result of the LPSi → BD*X–Y and LPX → LP*Si orbital interactions. Generally, the pnicogen bond becomes stronger in the order of F < Cl < Br < I and weaker in the order of P > As > Sb, exhibiting an unexpected substitution effect and dependence on the nature of the pnicogen atom. These orders are inconsistent with the MEP on the X atom but can be better explained by the above orbital interactions. The Si⋯X interaction displays a character of covalent or partially covalent interaction, evidenced by the high interaction energy of −59.9 to −105.4 kJ mol−1 as well as the negative energy density and the great charge transfer.

 Please wait while we load your content...
Please wait while we load your content...