Effect of hydration on the organo-noble gas molecule HKrCCH: role of krypton in the stabilization of hydrated HKrCCH complexes†
Abstract
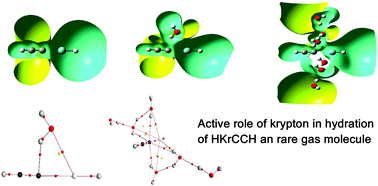
The effect of hydration on the fluorine free organo-noble gas compound HKrCCH and the role of krypton in the stabilization of the hydrated HKrCCH complexes have been investigated using the quantum chemical calculations on the HKrCCH–(H2O)n=1–6 clusters. Structure and energetics calculations show that water stabilizes HKrCCH through the π hydrogen bond in which the OH group of water interacts with the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C group of HKrCCH. A maximum of four water molecules can directly interact with the C
C group of HKrCCH. A maximum of four water molecules can directly interact with the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C of HKrCCH and after that only inter-hydrogen bonding takes place between the water molecules indicating that the primary hydration shell contains four water molecules. Atom in molecule analysis depicts that π hydrogen bonded complexes of the hydrated HKrCCH are cyclic structures in which the O⋯Kr interaction cooperates in the formation of strong O–H⋯C
C of HKrCCH and after that only inter-hydrogen bonding takes place between the water molecules indicating that the primary hydration shell contains four water molecules. Atom in molecule analysis depicts that π hydrogen bonded complexes of the hydrated HKrCCH are cyclic structures in which the O⋯Kr interaction cooperates in the formation of strong O–H⋯C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C interaction. Structure, energetics and charge analysis clearly established that krypton plays an important role in the stabilization as well as the formation of the primary hydration shell of hydrated HKrCCH complexes.
C interaction. Structure, energetics and charge analysis clearly established that krypton plays an important role in the stabilization as well as the formation of the primary hydration shell of hydrated HKrCCH complexes.

 Please wait while we load your content...
Please wait while we load your content...