Self-catalytic polymerization of a water-soluble selenium/polypyrrole nanocomposite and its nonlinear optical properties
Abstract
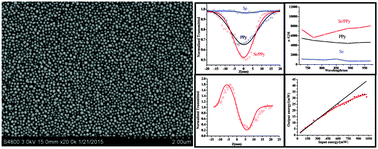
A facile one-step method for the synthesis of a water-soluble selenium/polypyrrole (Se/PPy) nanocomposite was developed. In the aqueous synthesis process, the pyrrole acted as a reductant for the reduction of H2SeO3, and then the elemental Se formed in situ acted as a catalyst for the polymerization of pyrrole. The characterization results show that the as-obtained composite (Se/PPy) is a spherical (Φ80 nm) product that is made up of amorphous Se particles coated by PPy layers. The formation mechanism and influence factors of the products were discussed, based on a series of experiments. It is proposed that remainder H2SeO3 adsorbed on the PPy chains increased the water-solubility and conductivity of the Se/PPy nanocomposite. Significantly, relying on the synergistic effect of photo-conductive Se nanoparticles and electric-conductive PPy molecules, the Se/PPy nanocomposite possesses a large two-photon absorption (2PA) cross-section and good optical limiting properties, which were demonstrated by the Z-scan technique using a femtosecond laser. We believe that this work should be an interesting strategy for developing polymer composites with excellent optoelectrical properties.

 Please wait while we load your content...
Please wait while we load your content...