Combined near-infrared excited SEHRS and SERS spectra of pH sensors using silver nanostructures†
Abstract
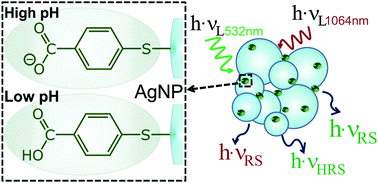
Surface-enhanced hyper-Raman scattering (SEHRS) and surface-enhanced Raman scattering (SERS) of para-mercaptobenzoic acid (pMBA) were studied with an excitation wavelength of 1064 nm, using different silver nanostructures as substrates for both SEHRS and SERS. The spectra acquired for different pH values between pH 2 and pH 12 were compared with SERS data obtained from the identical samples at 532 nm excitation. Comparison of the ratios of the enhancement factors from SEHRS and SERS experiments with those from calculations using plasmonic absorbance spectra suggests that the difference between total surface-enhancement factors of SEHRS and SERS for pMBA is mainly explained by a difference between the electromagnetic contributions for linear and non-linear SERS. SERS and SEHRS spectra obtained at near-infrared (NIR) excitation indicate an overall reduction of enhancement by a factor of 2–3 at very low and very high pH, compared to neutral pH. Our data provide evidence that different molecular vibrations and/or different adsorption species are probed in SERS and SEHRS, and that SEHRS is very sensitive to slight changes in the pMBA–nanostructure interactions. We conclude that the combination of SEHRS and SERS using NIR excitation is more powerful for micro-environmental pH sensing than one-photon spectra excited in the visible range alone.

 Please wait while we load your content...
Please wait while we load your content...