Toward the design of alkynylimidazole fluorophores: computational and experimental characterization of spectroscopic features in solution and in poly(methyl methacrylate)†
Abstract
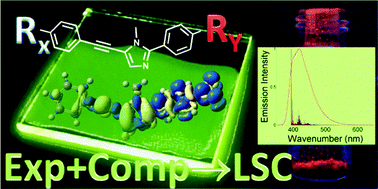
The possibilities offered by organic fluorophores in the preparation of advanced plastic materials have been increased by designing novel alkynylimidazole dyes, featuring different push and pull groups. This new family of fluorescent dyes was synthesized by means of a one-pot sequential bromination–alkynylation of the heteroaromatic core, and their optical properties were investigated in tetrahydrofuran and in poly(methyl methacrylate). An efficient in silico pre-screening scheme was devised as consisting of a step-by-step procedure employing computational methodologies by simulation of electronic spectra within simple vertical energy and more sophisticated vibronic approaches. Such an approach was also extended to efficiently simulate one-photon absorption and emission spectra of the dyes in the polymer environment for their potential application in luminescent solar concentrators. Besides the specific applications of this novel material, the integration of computational and experimental techniques reported here provides an efficient protocol that can be applied to make a selection among similar dye candidates, which constitute the essential responsive part of those fluorescent plastic materials.

 Please wait while we load your content...
Please wait while we load your content...