Removal of multi-substituted nitroaromatic pollutants by zero valent iron: a comparison of performance, kinetics, toxicity and mechanisms†
Abstract
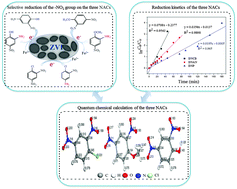
Reductive degradation of three typical multi-substituted nitroaromatic pollutants by zero valent iron was comprehensively compared in terms of performance, kinetics, toxicity and mechanisms in this study. The results showed that 0.5 mM 2,4-dinitrochlorobenzene (DNCB), 2,4-dinitroanisole (DNAN) and 2,4-dinitrophenol (DNP) could be completely removed in the ZVI reduction system within 75 min, 90 min and 210 min, respectively. The pseudo first-order kinetics could well describe the reduction process of the three NACs by ZVI. The reduction rates of the three NACs follow the order of DNCB > DNAN > DNP, which was further confirmed by density functional theory computational analysis. Moreover, the acute toxicity of the three NAC effluents significantly decreased after treatment with ZVI. In addition, the mechanistic investigation revealed that the selective reduction of nitro groups on the three NACs was closely related to the characteristics of the functional groups on the benzene rings. The results of this study would increase the comprehensive understanding in terms of their performance, kinetics, toxicity and mechanisms involved in the reduction of multi-substituted NACs by ZVI, thus benefiting the effective treatment of wastewaters containing multi-substituted nitroaromatic pollutants due to ZVI.

 Please wait while we load your content...
Please wait while we load your content...