Nanoparticles in ionic liquids: interactions and organization
Abstract
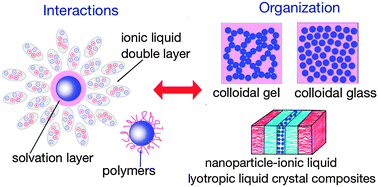
Ionic liquids (ILs), defined as low-melting organic salts, are a novel class of compounds with unique properties and a combinatorially great chemical diversity. Ionic liquids are utilized as synthesis and dispersion media for nanoparticles as well as for surface functionalization. Ionic liquid and nanoparticle hybrid systems are governed by a combined effect of several intermolecular interactions between their constituents. For each interaction, including van der Waals, electrostatic, structural, solvophobic, steric, and hydrogen bonding, the characterization and quantitative calculation methods together with factors affecting these interactions are reviewed here. Various self-organized structures based on nanoparticles in ionic liquids are generated as a result of a balance of these intermolecular interactions. These structures, including colloidal glasses and gels, lyotropic liquid crystals, nanoparticle-stabilized ionic liquid-containing emulsions, ionic liquid surface-functionalized nanoparticles, and nanoscale ionic materials, possess properties of both ionic liquids and nanoparticles, which render them useful as novel materials especially in electrochemical and catalysis applications. This review of the interactions within nanoparticle dispersions in ionic liquids and of the structure of nanoparticle and ionic liquid hybrids provides guidance on the rational design of novel ionic liquid-based materials, enabling applications in broad areas.

 Please wait while we load your content...
Please wait while we load your content...