Electrochemical and ab initio investigations to design a new phenothiazine based organic redox polymeric material for metal-ion battery cathodes
Abstract
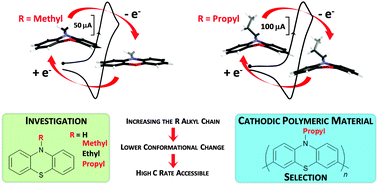
Different N-substituted phenothiazines have been synthesized and their electrochemical behavior has been investigated in CH3CN in order to design the best polyphenothiazine based cathodic material candidate for lithium batteries. These compounds exhibit two successive reversible one-electron oxidation processes. Ab initio calculations demonstrate that the potential of the first process is a result of both the hybridization effects between the substituent and the phenothiazine unit as well as the change of conformation of the phenothiazine heterocycle during the oxidation process. More specifically, we show that an asymmetric molecular orbital spreading throughout an external cycle of the phenothiazine unit and the alkyl fragment is formed only if the alkyl fragment is long enough (from the methyl moiety onwards) and is at the origin of the bent conformation for N-substituted phenothiazines during oxidation. Electrochemical investigations supported by ab initio calculations allow the selection of a phenothiazinyl unit which is then polymerized by a Suzuki coupling strategy to avoid the common solubilization issue in carbonate-based liquid electrolytes of lithium cells. The first electrochemical measurements performed show that phenothiazine derivatives pave the way for a promising family of redox polymers intended to be used as organic positives for lithium batteries.

 Please wait while we load your content...
Please wait while we load your content...