Statistical modeling of the reactions Fe+ + N2O → FeO+ + N2 and FeO+ + CO → Fe+ + CO2
Abstract
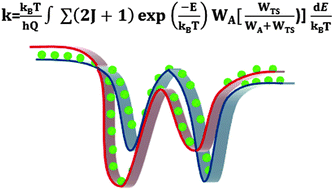
The rates of the reactions Fe+ + N2O → FeO+ + N2 and FeO+ + CO → Fe+ + CO2 are modeled by statistical rate theory accounting for energy- and angular momentum-specific rate constants for formation of the primary and secondary cationic adducts and their backward and forward reactions. The reactions are both suggested to proceed on sextet and quartet potential energy surfaces with efficient, but probably not complete, equilibration by spin-inversion of the populations of the sextet and quartet adducts. The influence of spin-inversion on the overall reaction rate is investigated. The differences of the two reaction rates mostly are due to different numbers of entrance states (atom + linear rotor or linear rotor + linear rotor, respectively). The reaction Fe+ + N2O was studied either with 6Fe+ or with 4Fe+ reactants. Differences in the rate constants of 6Fe+ and 4Fe+ reacting with N2O are attributed to different contributions from electronically excited potential energy surfaces, such as they originate from the open-electronic shell reactants.

 Please wait while we load your content...
Please wait while we load your content...